Creutzfeldt-Jakob Disease Market - Comprehensive Overview of End Users
The Creutzfeldt-Jakob Disease (CJD) market is gradually expanding as advancements in neurology, diagnostics, and palliative care continue to improve disease management and patient outcomes. CJD is a rare, fatal neurodegenerative disorder caused by prion protein misfolding, leading to rapid brain deterioration and severe neurological decline. It presents through cognitive impairment, memory loss, behavioral changes, and motor dysfunction, typically progressing within months.
While the disease remains incurable, growing research efforts, improved diagnostic tools, and early intervention strategies have strengthened healthcare system’s ability to manage and monitor cases effectively. Furthermore, the market is driven by innovation across diagnostics, genetic testing, surveillance, and palliative care services each involving multiple end users who play vital roles in advancing patient support and scientific understanding.
This blog explores the key end-user segments shaping the Creutzfeldt-Jakob Disease market, highlighting how hospitals, diagnostic centers, research institutions, and others contribute to the evolving landscape of prion disease care.
- Hospitals and Specialty Neurology Centers
Hospitals and specialized neurology centers remain the cornerstone of CJD diagnosis, treatment, and patient care. Given the disease’s complex neurological nature, hospitals serve as primary hubs for both clinical assessment and symptomatic management.
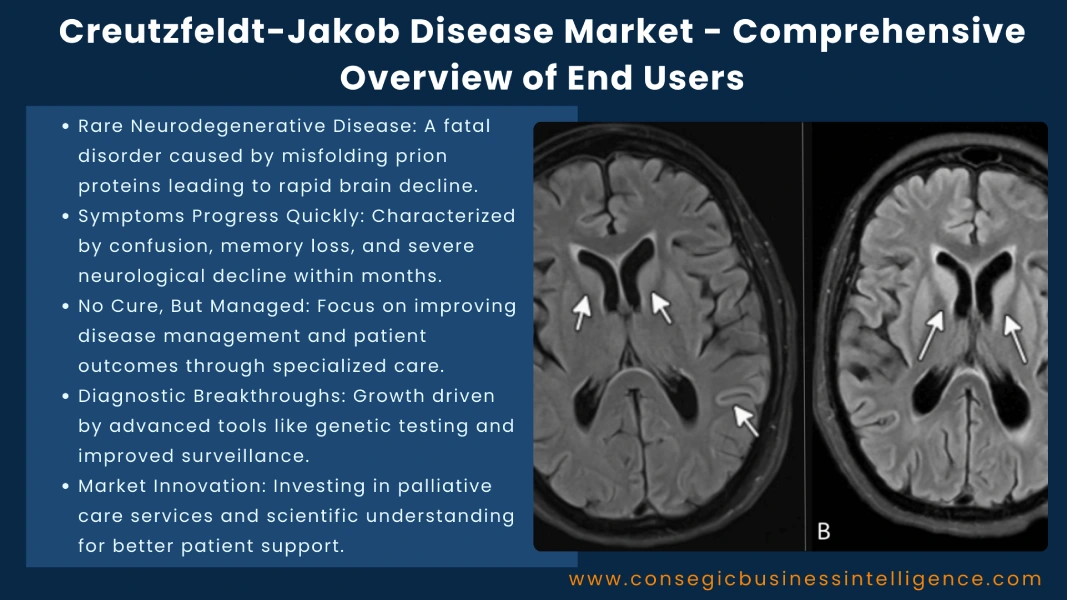
Modern healthcare institutions are adopting advanced imaging tools such as magnetic resonance imaging (MRI) and electroencephalography (EEG) to detect brain changes associated with prion activity. Many tertiary care hospitals have established neurology departments equipped with dedicated prion surveillance units to support early detection and case documentation.
Hospitals also play a critical role in implementing multidisciplinary care models, bringing together neurologists, psychiatrists, and palliative specialists to address both physical and cognitive symptoms. With the integration of electronic health records (EHRs) and tele-neurology platforms, hospitals are now better equipped to collaborate with global prion research networks.
As the demand for early diagnosis and compassionate care rises, hospitals continue to anchor the CJD market through their focus on comprehensive management and clinical coordination.
- Diagnostic and Imaging Centers
Diagnostic and imaging centers are among the most vital end users in the CJD market, providing accurate testing and advanced biomarker-based detection. Since confirming a CJD diagnosis is complex, these facilities utilize sophisticated diagnostic tools to detect hallmark signs of the disease.
Leading centers now perform tests like cerebrospinal fluid (CSF) assays, which analyze biomarkers such as 14-3-3 and tau proteins, and the real-time quaking-induced conversion (RT-QuIC) test, which detects abnormal prion proteins with exceptional sensitivity. Diagnostic imaging, particularly diffusion-weighted MRI, helps identify characteristic cortical and basal ganglia changes, supporting differential diagnosis from other neurological disorders.
The growing availability of automated laboratory platforms and next-generation immunoassays is improving diagnostic accuracy and reducing turnaround time. As healthcare providers emphasize early and reliable diagnosis, diagnostic centers are investing in new technologies and partnerships with research laboratories, enhancing their role within the broader prion disease ecosystem.
- Research and Academic Institutions
Research and academic institutions represent a critical end-user segment driving innovation in the CJD market. Their work focuses on understanding disease mechanisms, developing therapeutic interventions, and improving detection methods.
Universities and medical schools conduct molecular and genetic research to study the behavior of prion proteins, exploring how structural misfolding leads to neuronal damage. Many research centers collaborate internationally to create CJD biobanks, allowing scientists to store and analyze brain tissue, blood, and CSF samples for long-term studies.
Institutions such as the National Prion Clinic (UK), Centers for Disease Control and Prevention (CDC), and European Creutzfeldt-Jakob Disease Surveillance Network (EUROCJD) are leading global efforts in disease surveillance and knowledge sharing. Additionally, academic partnerships with biotechnology firms are accelerating the development of anti-prion drugs, immunotherapies, and genetic screening technologies.
As funding for rare disease research increases, academic and research institutions continue to be pivotal in shaping the scientific foundation of the CJD market.
- Long-Term Care Facilities and Nursing Homes
Long-term care facilities and nursing homes are becoming increasingly important in the CJD market due to the disease’s rapid progression and need for continuous medical supervision. Patients with CJD often require round-the-clock nursing, palliative care, and psychological support, especially during late stages of the disease.
These facilities focus on symptom management and comfort-based care, using multidisciplinary approaches that include physical therapy, speech support, and emotional counseling. With improved training and awareness, nursing homes are better equipped to recognize prion-related symptoms and implement infection control measures to prevent transmission risks during caregiving activities.
The integration of telemedicine and remote patient monitoring tools has also enhanced care delivery, allowing specialists to consult caregivers in real time. As life expectancy and awareness of neurodegenerative disorders rise globally, the role of long-term care facilities in providing compassionate and structured support continues to grow.
- Genetic Testing and Counseling Centers
A subset of CJD cases known as familial Creutzfeldt-Jakob Disease (fCJD) is hereditary, caused by mutations in the PRNP gene. Genetic testing and counseling centers play a crucial role in identifying these mutations, helping families understand their genetic risk and make informed decisions.
With advancements in next-generation sequencing (NGS), genetic testing has become faster and more accessible, allowing for early identification and monitoring of at-risk individuals. Genetic counselors collaborate closely with neurologists to interpret test results and provide emotional and psychological support to patients and relatives.
Additionally, genetic centers are contributing to population screening studies and genotype-phenotype correlation research, which help refine diagnostic accuracy and therapeutic research. As awareness of genetic counseling increases, these centers are becoming a vital link in both patient care and preventive medicine within the CJD market.
- Public Health Organizations and Surveillance Programs
Public health organizations play a critical role in tracking, reporting, and preventing CJD cases globally. Institutions like the World Health Organization (WHO), CDC, and EUROCJD collaborate with national health authorities to establish surveillance systems that monitor disease incidence and potential variant forms of CJD.
These organizations focus on implementing infection control guidelines, particularly in surgical and blood transfusion contexts, where prion transmission risks are higher. They also conduct public awareness campaigns to improve healthcare workers’ knowledge of disease recognition and management.
By maintaining comprehensive registries and facilitating cross-border data sharing, public health agencies are not only improving disease tracking but also supporting global preparedness for prion-related conditions.
- Pharmaceutical and Biotechnology Companies
Pharmaceutical and biotechnology firms represent an emerging end-user segment as they drive innovation in drug discovery and therapeutic development. Although there is currently no approved cure, these companies are investing heavily in anti-prion drug candidates, monoclonal antibodies, and small molecule inhibitors designed to slow or prevent prion replication.
Collaborations between industry players and academic institutions have led to preclinical trials exploring compounds that stabilize normal prion protein structures or prevent their conversion into infectious forms. Advances in nanotechnology-based drug delivery systems and gene-silencing approaches are also being explored to improve brain targeting and therapeutic efficacy.
As regulatory support for rare disease research increases, pharmaceutical companies are expected to play a more prominent role in shaping the future of CJD treatment and prevention.
Conclusion
The Creutzfeldt-Jakob Disease market is supported by a wide network of end users each contributing to advancing diagnosis, care, and research for one of the world’s most challenging neurological disorders. From hospitals and diagnostic centers delivering timely clinical interventions to research institutions, public health agencies, and biotech firms driving innovation, the collaborative ecosystem is strengthening global readiness against prion diseases.
As advancements in genetic testing, neuroimaging, and therapeutic development continue, the integration among these end users will be vital for improving patient care and scientific understanding. Together, they form the backbone of a market that is gradually shifting from reactive management to proactive research, offering hope for more effective solutions in the years ahead.
