Global Acute Intermittent Porphyria Market to Reach USD 7,274.28 Million by 2031 | CAGR of 6.3%
Category : Pharmaceuticals | Published Date : Nov 2024 | Type : Press Release
Acute Intermittent Porphyria Market Scope & Overview:
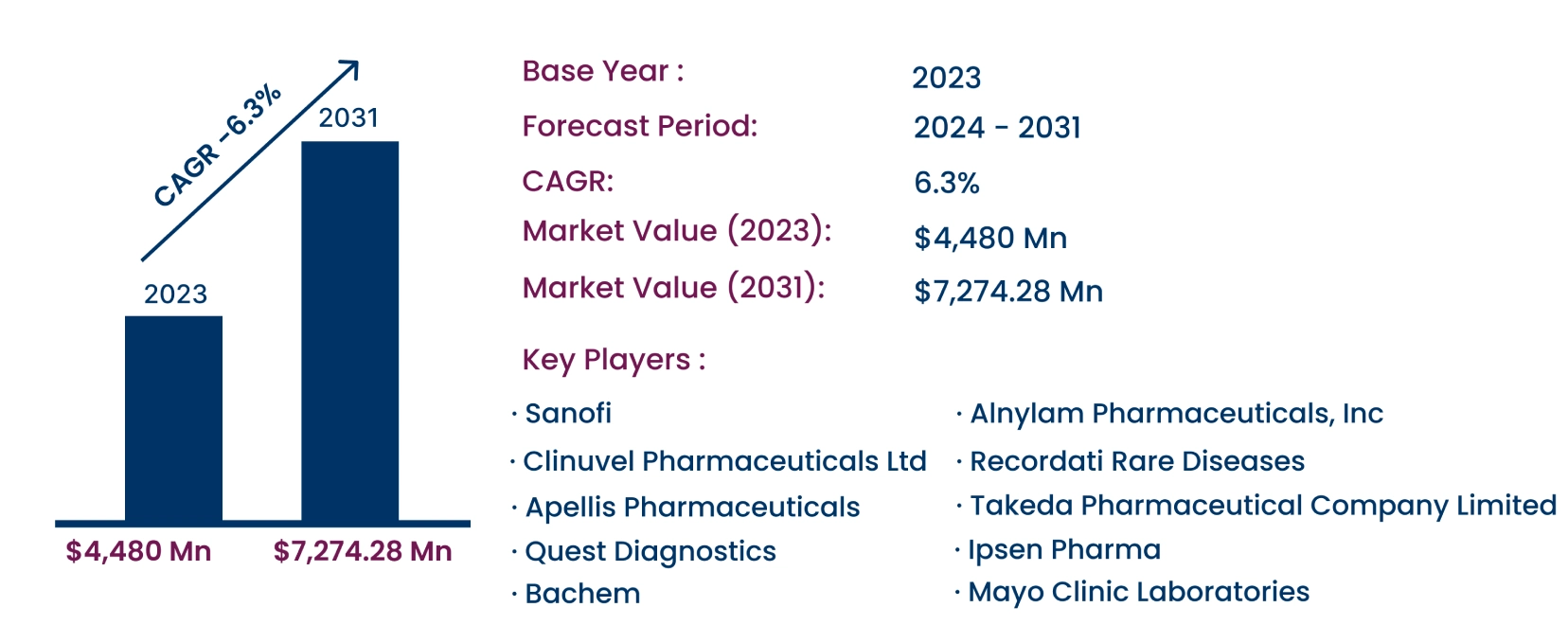
As per the Consegic Business Intelligence newly published report, the Acute Intermittent Porphyria Market was valued at USD 4,480.00 million in 2023 and is projected to grow at a CAGR of 6.3%, reaching USD 7,274.28 million by 2031. Acute intermittent porphyria (AIP) is a rare autosomal dominant genetic disorder characterized by a deficiency of hydroxymethylbilane synthase (HMBS). It manifests through symptoms such as abdominal pain, nausea, seizures, and peripheral neuropathy. Diagnostic methods include urine tests, genetic testing, and blood tests, with treatments ranging from intravenous heme therapy to RNA interference therapy.
The report comprises the Acute Intermittent Porphyria Market Share, Size & Industry Analysis, based on Type (Treatment (Gonadotropin-Releasing Hormone Analogs, Ribonucleic Acid Interference Therapy, Others), Diagnosis (Urine Tests, Genetic Testing, Others)), End-User (Hospitals, Clinics, Research Centers, Others), and Region (North America, Europe, Asia-Pacific, Latin America, Middle East & Africa), and Forecast, 2024-2031.
The report contains detailed information on Acute Intermittent Porphyria Market Trends, Opportunities, Value, Growth Rate, Segmentation, Geographical Coverage, Company Profiles, In-depth Expert Analysis, Revenue Forecast, Competitive Landscape, Growth Factors, Restraints or Challenges, Environment & Regulatory Landscape, PESTLE Analysis, PORTER Analysis, Key Technology Landscape, Value Chain Analysis, and Cost Analysis.
Advancements in treatments, such as RNA interference therapy, coupled with the growing adoption of genetic testing for precise diagnosis, are driving market growth. Additionally, collaborations between pharmaceutical companies and healthcare organizations present significant opportunities to raise awareness and improve treatment accessibility.
Segmental Analysis :
Based on type, the market is segmented into Treatment (Gonadotropin-Releasing Hormone Analogs, Ribonucleic Acid Interference Therapy, Others) and Diagnosis (Urine Tests, Genetic Testing, Others).
- The treatment segment accounted for the largest revenue share in 2023, driven by the widespread use of gonadotropin-releasing hormone analogs and hemin therapy for effective management of acute attacks.
- The diagnosis segment is projected to grow at the fastest CAGR, supported by the increasing adoption of genetic testing, which provides precise identification of HMBS mutations and facilitates preventive care.
Based on end-user, the market is segmented into Hospitals, Clinics, Research Centers, and Others.
- The hospitals segment held the largest revenue share of 46.72% in 2023, attributed to the availability of advanced diagnostic tools and specialized healthcare professionals offering comprehensive treatment.
- The clinics segment is expected to grow at the fastest CAGR, supported by their cost-effective and accessible services, facilitating early detection and disease management.
Based on regions, the market is segmented into North America, Europe, Asia-Pacific, Middle East & Africa, and Latin America.
- North America: Dominated the market in 2023, holding 40.11% of the global share, driven by advanced medical research, high adoption of RNA interference therapy, and improved access to FDA-approved treatments like Panhematin®.
- Europe: Expected to witness the fastest CAGR of 7.0% during the forecast period, supported by a higher prevalence of AIP, robust healthcare infrastructure, and strong awareness programs for early diagnosis and treatment.
| Report Attributes | Report Details |
| Study Timeline | 2018-2031 |
| Market Size in 2031 | USD 7,274.28 Million |
| CAGR (2024-2031) | 6.3% |
| Type | Treatment (Gonadotropin-Releasing Hormone Analogs, Ribonucleic Acid Interference Therapy, Others), Diagnosis (Urine Tests, Genetic Testing, Others) |
| End-User | Hospitals, Clinics, Research Centers, Others |
| By Region | North America(U.S., Canada, Mexico) Europe(U.K., Germany, France, Spain, Italy, Russia, Benelux, Rest of Europe) APAC(China, South Korea, Japan, India, Australia, ASEAN, Rest of Asia-Pacific) Middle East & Africa(GCC, Turkey, South Africa, Rest of MEA) LATAM(Brazil, Argentina, Chile, Rest of LATAM) |
Top Key Players & Competitive Landscape :
The competitive landscape encompasses major innovators, aftermarket service providers, industry giants, and niche players, all of which are thoroughly examined by Consegic Business Intelligence in terms of their strengths, weaknesses, and value-addition potential. This report includes detailed profiles of key players, market share analysis, mergers and acquisitions, resulting market fragmentation, and emerging partnership trends and dynamics.
List of prominent players in the Acute Intermittent Porphyria Industry:
- Alnylam Pharmaceuticals, Inc. (United States)
- Recordati Rare Diseases (Italy)
- Sanofi (France)
- Clinuvel Pharmaceuticals Ltd (Australia)
- Apellis Pharmaceuticals (United States)
- Quest Diagnostics (United States)
- Mayo Clinic Laboratories (United States)
- Bachem (Switzerland)
- Takeda Pharmaceutical Company Limited (Japan)
- Ipsen Pharma (France)
Recent Industry Developments :
- March 2024: Sun Pharma received approval from the Central Drug Standard Control Organization to study Leuprolide Acetate for managing AIP.
- April 2021: Alnylam launched an international observational registry (ELEVATE) to study the long-term safety and effectiveness of givosiran in AIP patients.
- September 2021: Givlaari® (givosiran), an RNA interference therapy, was made available for community-based treatment, enhancing accessibility for AIP patients.