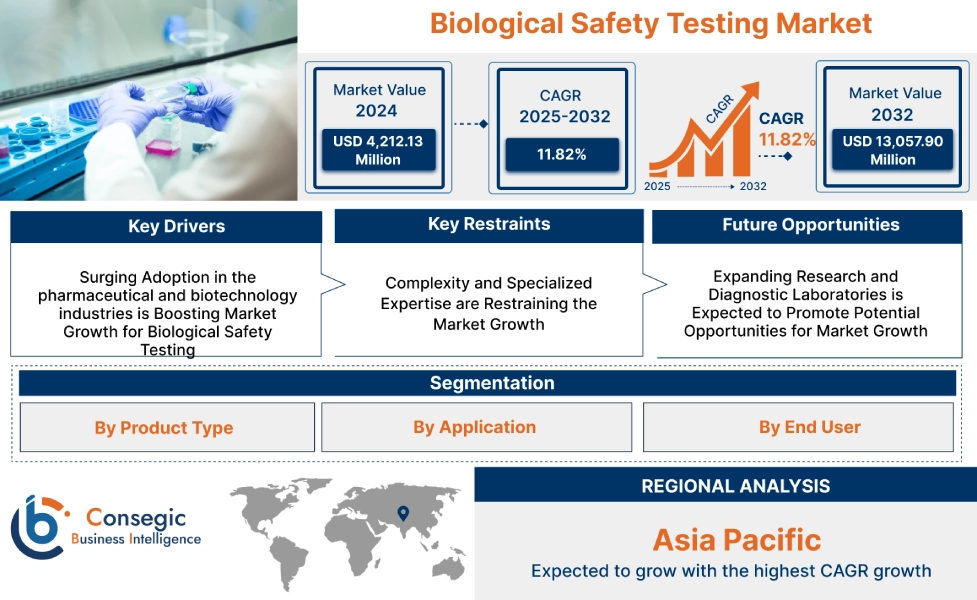
Biological Safety Testing Market Size:
Biological Safety Testing Market size is estimated to reach over USD 13,057.90 Million by 2032 from a value of USD 4,212.13 Million in 2024 and is projected to grow by USD 4,778.73 Million in 2025, growing at a CAGR of 11.82% from 2025 to 2032 .
Biological Safety Testing Market Scope & Overview:
Biological safety testing involves the verification of products such as medicines, medical devices, and laboratory materials. The main focus is to identify if the products are safe, effective, and free from contaminants, such as viruses, bacteria, and endotoxins, before sending them out in the market. Also, it is a crucial aspect of ensuring public health and regulatory compliance, by utilizing a range of methods from sterility and mycoplasma testing to examinations for agents is driving the biological safety testing market demand. Additionally, the key advantages include improved product quality, reduced patient risk, environmental protection, cost and time efficiency, among others. The aforementioned benefits are major determinants for increasing their deployment in pharmaceutical, biotechnology, and other industries. Further, the Innovations in instrumentation, reagents, and software tools are enhancing the detection, which in turn is boosting the biological safety testing market growth.
How is AI Impacting the Biological Safety Testing Market?
Artificial Intelligence (AI) and machine learning algorithms can analyze complex data quickly and efficiently, which in turn leads to improved accuracy in stability tests of impurities and contaminants. Also, AI optimizes lab processes, shortens testing timelines, and reduces human error, making biologics production more efficient and cost-effective. Additionally, AI facilitates the creation of specialized high-precision testing protocols to adapt to the rise of complex cell and gene therapies. Further, AI-powered platforms and tools aid in maintaining compliance with stringent regulations by providing real-time monitoring and insights for decision making in drug development is fueling the biological safety testing industry.
Biological Safety Testing Market Dynamics - (DRO) :
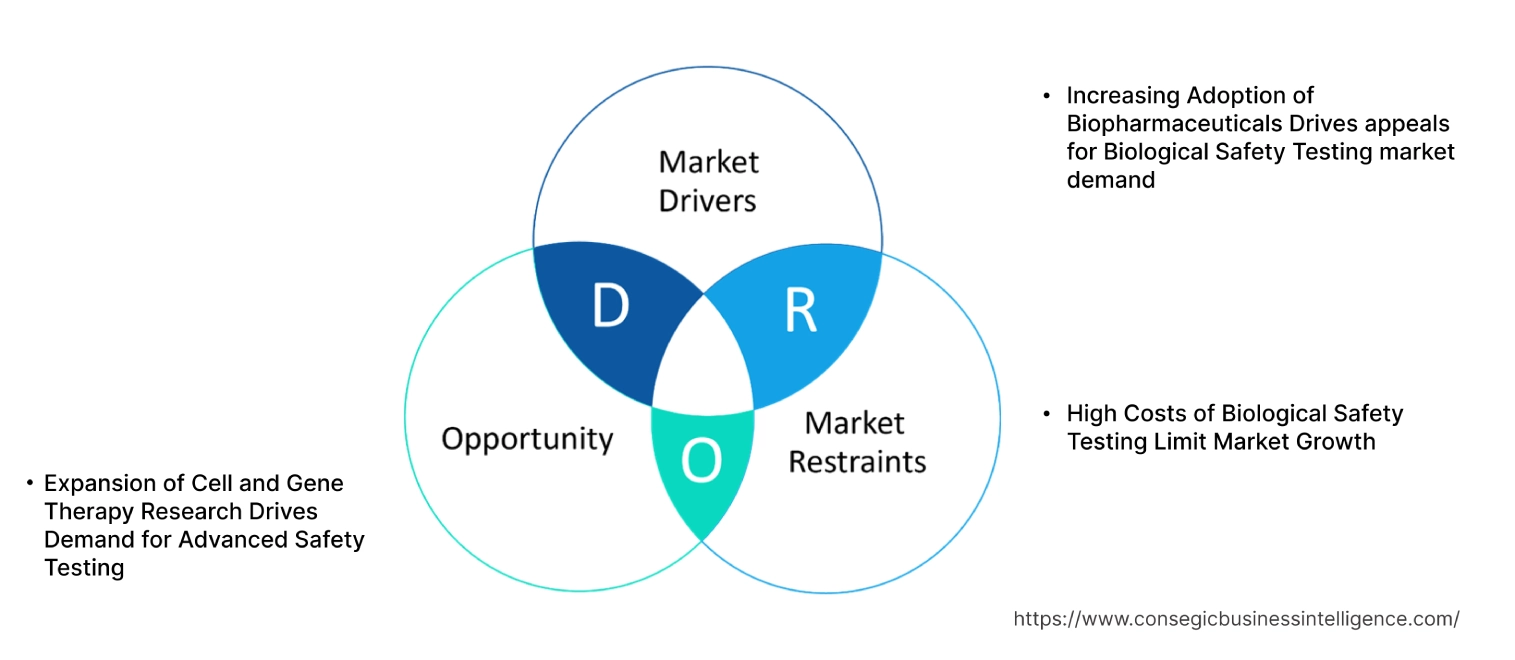
Key Drivers:
Surging Adoption in the pharmaceutical and biotechnology industries is Boosting Market Growth for Biological Safety Testing
The expanding pharmaceutical and biotechnology sector, which includes vaccines, antibodies, and gene/cell therapies (CGT), is driving the demand for comprehensive safety testing to ensure product quality and regulatory compliance. Also, regulatory bodies are implementing stricter guidelines for novel and advanced therapies that drive the biological safety testing market demand. Further, Pharmaceutical and biotech companies are increasingly outsourcing safety testing to specialized firms, which maintaining high safety standards is driving the biological safety testing market growth.
- For instance, in April 2024, Merck launched a genetic stability assay platform designed for biotech companies. The platform aims to address genetic stability requirements. The platform significantly accelerates biosafety testing for clients as well as provides next-generation sequencing technology, which reduces testing time by 66% and reduces costs by 43% compared to traditional methods.
Therefore, the growth of the pharmaceutical and biotechnology sector is driving the adoption of testing equipment, in turn, proliferating the growth of the market.
Key Restraints :
Complexity and Specialized Expertise are Restraining the Market Growth
Advanced biological safety testing requires costly instruments, cleanrooms, and other specialized laboratory infrastructure, which in turn creates a financial barrier for many biopharmaceutical companies. Also, the test procedures are complex, which requires skilled professionals and is significantly increasing the operational cost of equipment and is hindering the biological safety testing market expansion. Further, the rapidly evolving nature of testing methods requires constant upskilling and retraining of personnel, which adds to the cost and time. Furthermore, the shortage of qualified employees with specialized knowledge and experience can hinder the market's progress.
Therefore, the increased complexity of equipment and the need for specialized expertise add up to the operational cost, which in turn hinders the biological safety testing market expansion.
Future Opportunities :
Expanding Research and Diagnostic Laboratories is Expected to Promote Potential Opportunities for Market Growth
The increasing need for safety testing services and products, due to advancements in biologics and rising chronic diseases, is driving the need for laboratories, as well as the rising adoption of advanced technologies, such as next-generation sequencing (NGS) and AI, is paving the way for biological safety testing market opportunities. Further, the increasing prevalence of chronic diseases fuels demand for biopharmaceuticals is driving the need for robust testing methods. Moreover, many pharmaceutical and biopharmaceutical companies are increasingly outsourcing safety testing to specialized firms, which in turn is boosting the market development.
- For instance, in March 2025, according to the Indian Ministry of Health and Family Welfare, the government has approved setting up 165 bio-safety laboratories, which include 11 BSL-3 level labs and 154 BSL-2 level labsfor managing epidemics and national calamities.
Hence, the rising number of bio-safety laboratories is anticipated to increase the utilization of advanced equipment’s in turn promoting prospects for the biological safety testing market opportunities during the forecast period.
Biological Safety Testing Market Segmental Analysis :
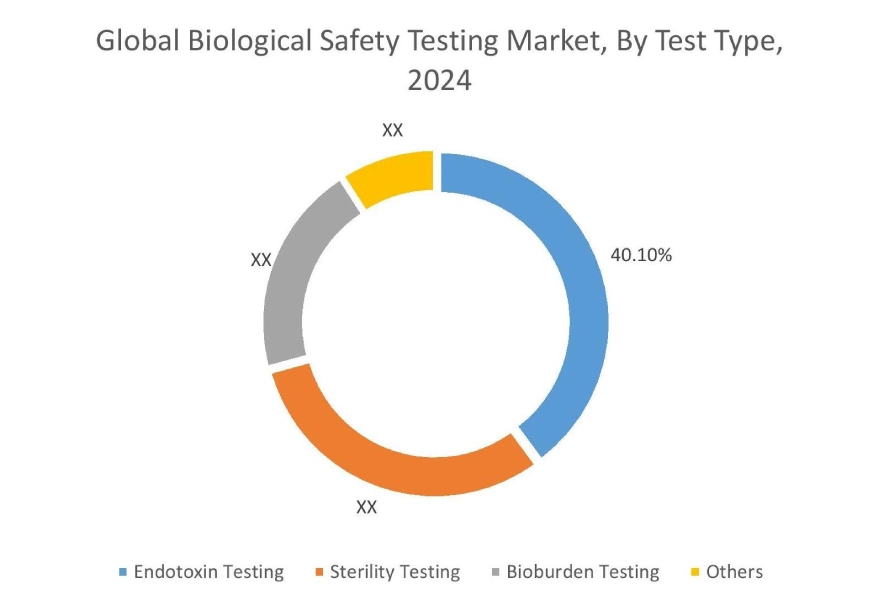
By Test Type:
Based on the test type, the market is segmented into endotoxin testing, sterility testing, bioburden testing, and others
Trends in the Test Type:
- The integration of sterility testing with rapid microbiological methods (RMMs) to reduce test times is driving the biological safety testing market trends.
- The rise of automation and non-animal assays for endotoxin testing is driving the biological safety testing market trends.
The endotoxin testing accounted for the largest revenue share of 40.10% in the year 2024.
- Endotoxin testing is a critical component of safety testing, specifically designed to focus on detecting and measuring lipopolysaccharides from the cell walls of gram-negative bacteria in pharmaceutical, biological, and medical device products.
- Also, the test is essential due to endotoxins are potent pyrogens when they enter the bloodstream, which can cause severe reactions like fever, shock, and organ failure, which in turn is fueling the biological safety testing market share.
- Additionally, the key advantages include patient safety, enhanced product quality, early detection, and others.
- Further, the stricter regulatory requirements and the enlargement of the biotechnology sector are driving the need for endotoxin tests, which in turn is fueling the biological safety testing market share.
- For instance, in January 2024, Charles River Laboratories International, Inc. launched a rapid animal-free bacterial endotoxin test, which enhances test efficiency and accelerates manufacturing timelines.
- Thus, as per the biological safety testing market analysis, the stricter regulatory requirements, as well as the enlargement of the biotechnology sector, are driving the market progress.
The sterility testing is anticipated to register the fastest CAGR during the forecast period.
- Sterility testing confirms a product is free of viable microorganisms, crucial for patient safety in sterile pharmaceuticals and medical devices. The methods used include membrane filtration or direct inoculation, with incubation for 14 days to ensure patient safety.
- Also, sterility testing is a key component of quality control in pharmaceutical and medical device manufacturing, ensuring products are sterile for use.
- Further, the need to ensure patient and product safety under strict regulatory frameworks, as well as the rising prevalence of chronic and infectious diseases, is driving the need for sterility testing, which in turn is boosting the biological safety testing market size.
- Therefore, as per the market analysis, the increased throughput, reduced costs, and strict regulatory frameworks are anticipated to boost the market during the forecast period.
By Application:
Based on the application, the market is segmented into vaccine development, blood & blood products testing, gene therapy, cellular therapy, and others.
Trends in the Application:
- The trend towards the integration of AI and digital tools for risk assessment in gene therapy is boosting the market development.
- The growing trend toward adopting in vitro testing methods, such as cell-based assays, due to their speed, reduced reliance on animal testing, and cost-efficiency, is boosting the market progress.
Vaccine Development accounted for the largest revenue share in the year 2024.
- Vaccine development is a multi-stage process, from initial research to market approval, which involves difficult safety testing to ensure a vaccine is both safe and effective in humans.
- Also, the rigorous testing ensures the vaccine is pure, free of contaminants, and consists of the correct biological components.
- Further, the rising expenditure on R&D activities is boosting the market adoption in vaccine development is driving the biological safety testing market size.
- Moreover, the rise of new vaccines for emerging infectious diseases continues to be developed, driving the need for safety testing equipment for vaccine development.
- For instance, in June 2025, according to the World Health Organization (WHO), due to a rapidly ageing population WHO has launched under Immunization Agenda 2030, a global strategy for vaccination, which in turn is propelling kids and adults to take vaccines against diseases such as influenza, COVID-19, pneumococcal disease, tetanus, and others.
- Thus, as per the biological safety testing market analysis, the rise of new vaccines for emerging infectious diseases is driving the market progress.
Gene Therapy is anticipated to register the fastest CAGR during the forecast period.
- Gene therapy has potential risks, such as immune reactions or off-target effects, which drive the need for rigorous safety testing. This essential testing process ensures purity, identity, potency, and freedom from contamination.
- Also, the testing is essential for gene therapies, as it ensures these powerful treatments are safe for patients by identifying and mitigating potential risks before and during their clinical application.
- Moreover, the rigorous testing process supports the safe and timely development and commercialization of novel gene therapies.
- Further, the rapid evolution and increased adoption of gene therapies are creating a significant demand for precision safety testing services.
- Therefore, as per the market analysis, the rising safety as well as evolution and increased adoption of gene therapies are anticipated to boost the market during the forecast period.
By End-User:
Based on the End User, the market is segmented into pharmaceutical & biotechnology companies, contract research organizations (CROs), academic & research institutes, medical device manufacturers, and others.
Trends in the End User:
- The trend towards growing emphasis on eco-friendly practices, such as green chemistry and waste reduction, is driving the adoption by medical device manufacturers.
- The trend towards increasing research and development of new therapies is driving the adoption of safety testing equipment by academic & research institutes.
The pharmaceutical & biotechnology companies accounted for the largest revenue share in the year 2024.
- Pharmaceutical and biotechnology companies rely on these rigorous tests to comply with stringent global regulations. Also, the safety testing ensures the quality and safety of vaccines, gene therapies, and other biologic drugs.
- Also, the key benefits include enhanced patient safety, regulatory compliance to gain market approval, risk mitigation, and protecting brand reputation, among others.
- Further, the growing global awareness of the importance of quality control in the healthcare industry, as well as the rising prevalence of infectious diseases, is driving the market adoption in pharmaceutical and biotechnology companies.
- Thus, as per the market analysis, the aforementioned factors are driving the market progress.
The contract research organizations (CROs) are anticipated to register the fastest CAGR during the forecast period.
- Contract research organizations (CROs) are specialized companies that provide research and development services. Also, they conduct a wide range of non-clinical and clinical research, ensuring drug and device safety, efficacy, and regulatory compliance for clients.
- Additionally, the key advantages include access to specialized expertise and advanced technology, leading to faster, more cost-effective, and higher-quality results.
- Further, the rising adoption of outsourcing services to ensure product safety and efficacy is driving the need for contract research organizations.
- Therefore, as per the market analysis, the aforementioned factors are anticipated to boost the market during the forecast period.
Regional Analysis:
The regions covered are North America, Europe, Asia Pacific, Middle East and Africa, and Latin America.
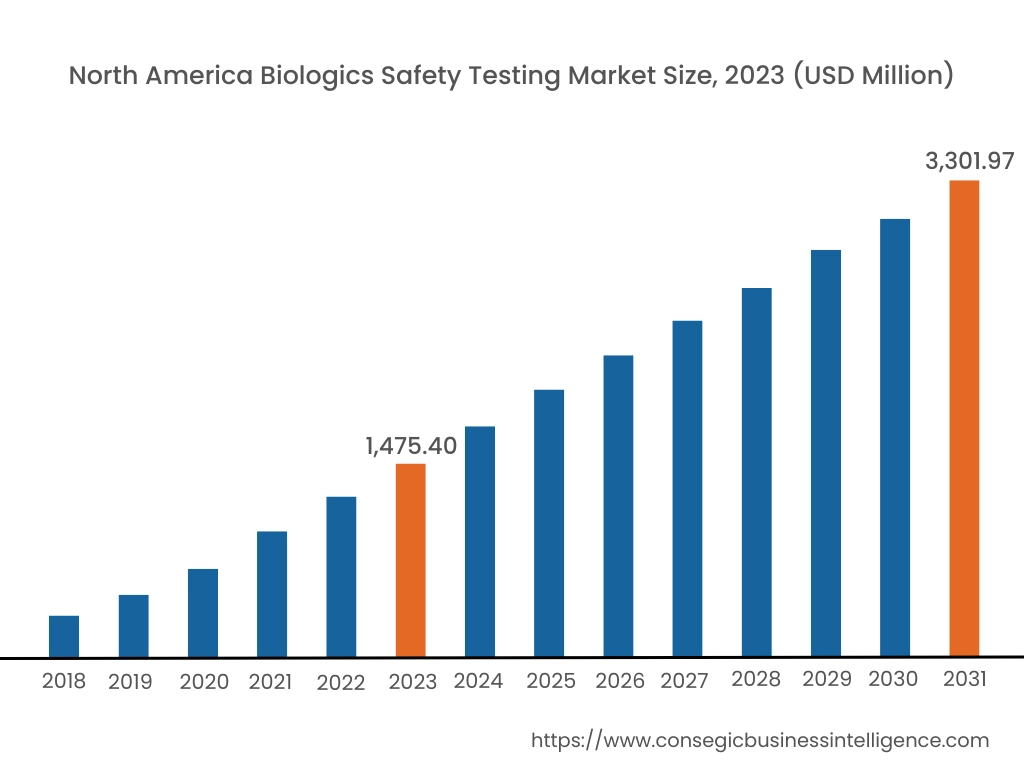
Asia Pacific region was valued at USD 1,126.31 Million in 2024. Moreover, it is projected to grow by USD 1,281.17 Million in 2025 and reach over USD 3,601.37 Million by 2032. Out of this, China accounted for the maximum revenue share of 34.30%. The market progress is mainly driven by the growing number of chronic diseases that create a need for new treatments. Furthermore, factors including the increased R&D and investment in biological drugs and advanced therapies like cell and gene therapies are projected to drive the market progress in the Asia Pacific region during the forecast period.
- For instance, in August 2025, IIT Delhi launched a new Biosafety Level 3 research facility for academic and industry scientists to advance research on highly infectious, class-3 pathogens and improve biomedical and clinical diagnostics in India.
North America is estimated to reach over USD 4,251.65 Million by 2032 from a value of USD 1,360.50 Million in 2024 and is projected to grow by USD 1,544.55 Million in 2025. The North American region's robust biopharmaceutical sector, focused on R&D, offers lucrative growth prospects for the market. Additionally, the stringent regulatory frameworks from bodies like the FDA and others, as well as advances in scientific innovation and technology, and the growing need for testing in medical devices, are driving the market progress.
- For instance, in July 2024, Emergent BioSolutions Inc. received a contract of more than USD 250 million to deliver millions of doses of four medical countermeasures at the United States Department of Health and Human Services (HHS), which in turn is boosting the market evolution.
The regional analysis depicts that the significant investments in the pharmaceutical and biotechnology sectors are driving the market in Europe. Additionally, the key factor driving the market is the development of life sciences and biotechnology sectors, particularly in countries such as the UAE and Saudi Arabia, as well as the growing demand for vaccines, gene therapies, biosimilars, and other biologics is propelling the market adoption in the Middle East and African region. Further, the increased investments in vaccine and biologic production and expanding healthcare infrastructure are paving the way for the progress of the market in the Latin America region.
Top Key Players & Market Share Insights:
The global biological safety testing market is highly competitive with major players providing biological safety testing to the national and international markets. Key players are adopting several strategies in research and development (R&D), product innovation, and end user launches to hold a strong position in the biological safety testing industry. Key players in the biological safety testing market include-
- Charles River Laboratories (USA)
- Merck KGaA (Germany)
- Thermo Fisher Scientific Inc. (USA)
- WuXi AppTec (China)
- Samsung Biologics (South Korea)
- Cytovance Biologics (USA)
- Lonza Group Ltd. (Switzerland)
- Sartorius AG (Germany)
- Eurofins Scientific (Luxembourg)
- SGS S.A. (Switzerland)
- Cytovance Biologics (USA)
Recent Industry Developments :
Product launches
- In January 2205, Esco Aster and Esco Lifesciences Group launched a bioreactor system designed to meet human and animal biosafety levels 3 and 4 standards. The launch aims to enable the safe handling of high-risk pathogens in containment environments.
Expansion
- In August 2024, WuXi Biologics announced the launch of four manufacturing facilities and a biosafety testing center in China and has received Good Manufacturing Practice certificates from the European Medicines Agency.
Biological Safety Testing Market Report Insights :
| Report Attributes | Report Details |
| Study Timeline | 2018-2031 |
| Market Size in 2031 | USD 13,057.90 Million |
| CAGR (2024-2031) | 11.82% |
| By Test Type |
|
| By Application |
|
| By End-User |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
How big is the biological safety testing market? +
The biological safety testing market size is estimated to reach over USD 13,057.90 Million by 2032 from a value of USD 4,212.13 Million in 2024 and is projected to grow by USD 4,778.73 Million in 2025, growing at a CAGR of 11.82% from 2025 to 2032.
Which segmentation details are covered in the biological safety testing report? +
The biological safety testing report includes specific segmentation details for test type, application, end user, and regions.
Which is the fastest segment anticipated to impact the market growth? +
In the biological safety testing market, sterility testing is the fastest-growing segment during the forecast period due to the increased throughput, reduced costs, and strict regulatory frameworks.
Who are the major players in the biological safety testing market? +
The key participants in the biological safety testing market are Charles River Laboratories (USA), Merck KGaA (Germany), Lonza Group Ltd. (Switzerland), Sartorius AG (Germany), Eurofins Scientific (Luxembourg), SGS S.A. (Switzerland), Thermo Fisher Scientific Inc. (USA), WuXi AppTec (China), Samsung Biologics (South Korea), Cytovance Biologics (USA), and others.
What are the key trends in the biological safety testing market? +
The biological safety testing market is being shaped by several key trends including growing emphasis on eco-friendly practices, such as green chemistry and waste reduction, as well as the rise of automation and non-animal assays for endotoxins testing, and others are key trends driving the market.