Medical Elastomer Market Size:
The Medical Elastomer Market size is growing with a CAGR of 9.4% during the forecast period (2025-2032), and the market is projected to be valued at USD 14.22 Billion by 2032 from USD 6.95 Billion in 2024. Additionally, the market value for 2025 is attributed to USD 7.58 Billion.
Medical Elastomer Market Scope & Overview:
A medical elastomer is a specialized type of polymeric material characterized by its elastic properties, allowing it to deform significantly under stress and subsequently return to its original shape. These materials are distinguished by their adherence to stringent requirements for biocompatibility in medical applications, ensuring they are non-toxic and do not elicit adverse reactions when in contact with biological systems. Furthermore, elastomers developed for medical applications present high purity, chemical resistance to bodily fluids and drugs, and compatibility with various sterilization methods without degrading. These unique properties make elastomers indispensable in the manufacturing of a wide array of healthcare products, including flexible components in catheters and tubing, seals in medical devices, plungers in syringes, and various long-term or short-term implants.
Key Drivers:
Growing Demand for Medical Devices to Drive Medical Elastomer Market Growth.
As global healthcare infrastructure expands and technological advancements lead to more sophisticated medical equipment, the need for high-performance, biocompatible materials such as elastomers escalates. Factors such as an aging global population, the increasing prevalence of chronic diseases, and the widespread adoption of minimally invasive surgical procedures result in a greater requirement for flexible, durable, and safe components in applications from catheters and IV tubing to syringes, implants, and wearable health monitoring devices. This increasing utilization across the medical device spectrum ensures sustained and significant benefit for the medical elastomer market demand.
- For instance, according to data published by Medtech Europe, European medical device market has demonstrated consistent growth, averaging 5.7% annually over 2009-22.
Thus, as per the analysis, the growing market for such materials in medical devices is fueling significant medical elastomer market growth.
Key Restraints:
Stringent Regulations to Hinder Medical Elastomer Market Expansion.
Stringent regulations serve as a significant impediment to market medical elastomers demand owing to dominant application in the highly regulated medical device sector. These strict regulatory mandates, such as the European Union's Medical Device Regulation (MDR) and the U.S. FDA requirements, impose rigorous demands on material suppliers and device manufacturers. Compliance necessitates extensive and costly material testing, clinical trials, and meticulous documentation to ensure the biocompatibility, safety, and long-term performance of elastomers in critical applications including implants and surgical devices. Non-compliance results in severe penalties, including product recalls and market prohibition, which in turn elevates development costs, extends market entry timelines, and ultimately dampen innovation in the medical elastomer industry. As a result, the above-mentioned factors are limiting the medical elastomer market expansion.
Future Opportunities :
Development of Bio-based and Sustainable Elastomers to Create Medical Elastomer Market Opportunities.
With increasing global emphasis on environmental responsibility and circular economy principles, the healthcare sector is actively seeking materials that reduce its carbon footprint and address concerns related to the disposal of conventional plastics. Bio-based elastomers, derived from renewable resources offer a suitable solution by providing comparable performance to traditional elastomers while presenting a more sustainable origin. This aligns with corporate sustainability goals and also resonates with growing consumer demand for greener healthcare products, thus opening new avenues for innovation within the medical elastomer sector.
- For instance, in 2024, Avient Corporation highlighted its range of bio-based polymer solutions, including bio-derived thermoplastic elastomers with lower product carbon footprints to support sustainability in drug delivery.
Henceforth, the development of sustainable elastomers is creating lucrative medical elastomer market opportunities over the forecast period.
Medical Elastomer Market Segmental Analysis :
By Type:
Based on type, the market is categorized into thermoplastic elastomers and thermoset elastomers
Trends in Type:
- Trend towards thermoplastic elastomers as safer, phthalate-free, and latex-free alternatives in disposable medical devices is positively impacting the market.
- Trend of developing highly specialized silicone formulations for complex or unique medical device needs is rising.
The thermoplastic elastomers segment accounted for the largest medical elastomer market share in 2024 and is also expected to grow at the fastest CAGR over forecast period.
- Thermoplastic elastomers (TPEs) are a class of polymers that exhibit the elasticity of rubber while retaining the processing advantages of thermoplastics.
- Common types include thermoplastic polyurethanes (TPUs), styrenic block copolymers (SBCs), thermoplastic vulcanizates (TPVs), and plasticized polyvinyl chloride (PVC).
- Their growth is significantly driven by their cost-effectiveness, customization ease, and the increasing demand for phthalate-free and latex-free materials in disposable medical devices like tubing and IV bags.
- Furthermore, TPEs' recyclability and lower energy processing align with growing sustainability initiatives, while continuous advancements enhance their biocompatibility and sterilization resistance.
- For instance, in 2024, Teknor Apex expanded its Medalist portfolio of medical-grade thermoplastic elastomers with new grades specifically engineered for biopharmaceutical tubing applications, addressing the need for flexible, rubber-like, high-quality, and regulatory-compliant materials in the healthcare sector.
- Thus, as per the medical elastomer market analysis, the thermoplastic elastomers segment is dominating the medical elastomer market demand and is expected to grow in the forecast period.
By Application:
Based on application, the market is categorized into medical tubing, syringes, gloves, medical bags, implants, seals and gaskets, drug delivery systems, and others.
Trends in the Application:
- The development of smaller diameter, multi-lumen, and highly customized tubing, crucial for enhancing precision in minimally invasive surgical procedures is a key trend.
- A significant trend is driven by the increasing use of pre-filled syringes for biologics and vaccines, demanding high-purity elastomer plungers.
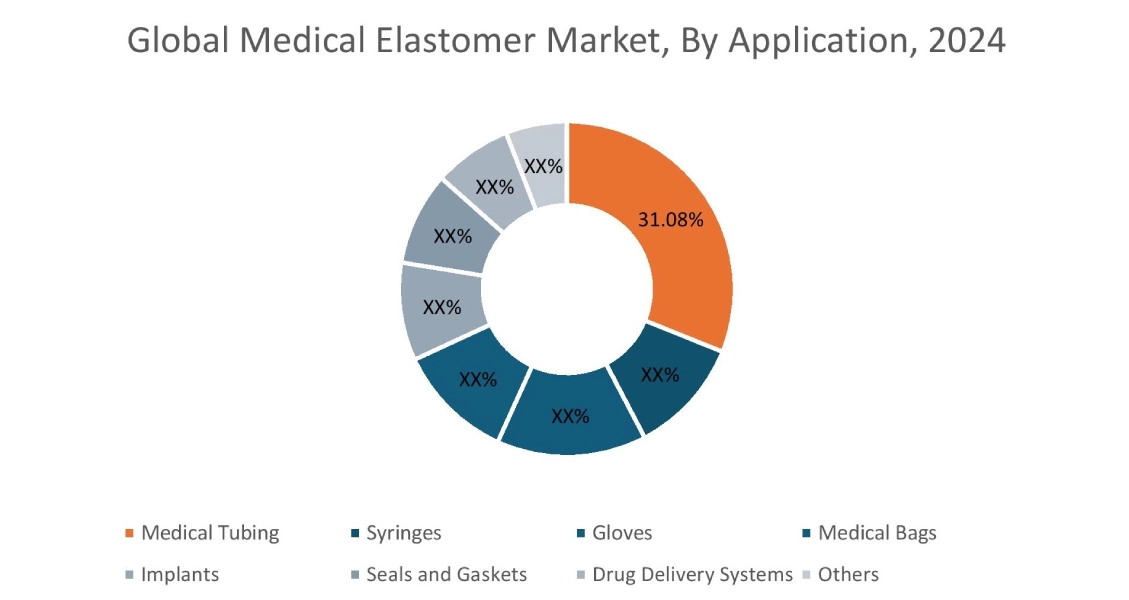
The medical tubing segment accounted for the largest Medical Elastomer Market share of 31.08% in 2024.
- Medical tubing consistently represents the largest application segment, encompassing a wide array of vital uses for fluid transfer, gas delivery, and drainage in healthcare settings.
- This includes critical components such as IV lines, various types of catheters, respiratory tubing, and drainage tubes.
- The segment revenue is significantly driven by the escalating number of surgical procedures performed globally, the expansion of home healthcare services, and the increasing prevalence of chronic diseases necessitating long-term care.
- For instance, according to Eurostat, 1.1 million transluminal coronary angioplasty procedures were conducted in the European Union Countries in 2021 and are expected to increase at a considerate growth over the forecast period.
- Thus, as per the aforementioned factors, the medical tubing segment is dominating market growth.
The implants segment is expected to grow at the fastest CAGR over the forecast period.
- The implants segment is a high-value and rapidly growing application area for medical elastomers, encompassing their use in both long-term and short-term devices.
- This includes critical components for breast implants, certain joint replacements, coatings for stents, drug-eluting implants, and temporary shunts.
- For these applications, significant importance is placed on the material's biocompatibility, ensuring it is inert within the body, along with its long-term stability and mechanical durability to withstand physiological stresses.
- The segment revenue is driven by increasing prevalence of chronic diseases, and advancements in smart implant technologies such as embedded sensors and personalized solutions.
- Thus, based on the market analysis, implants is the fastest growing segment.
Regional Analysis:
The regional segment includes North America, Europe, Asia Pacific, the Middle East and Africa, and Latin America.
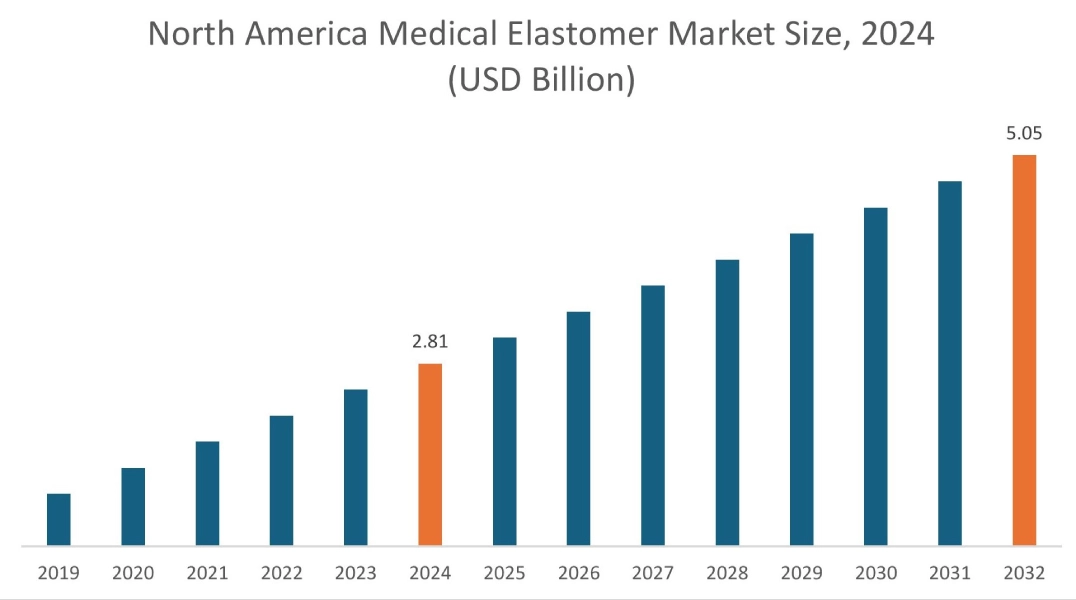
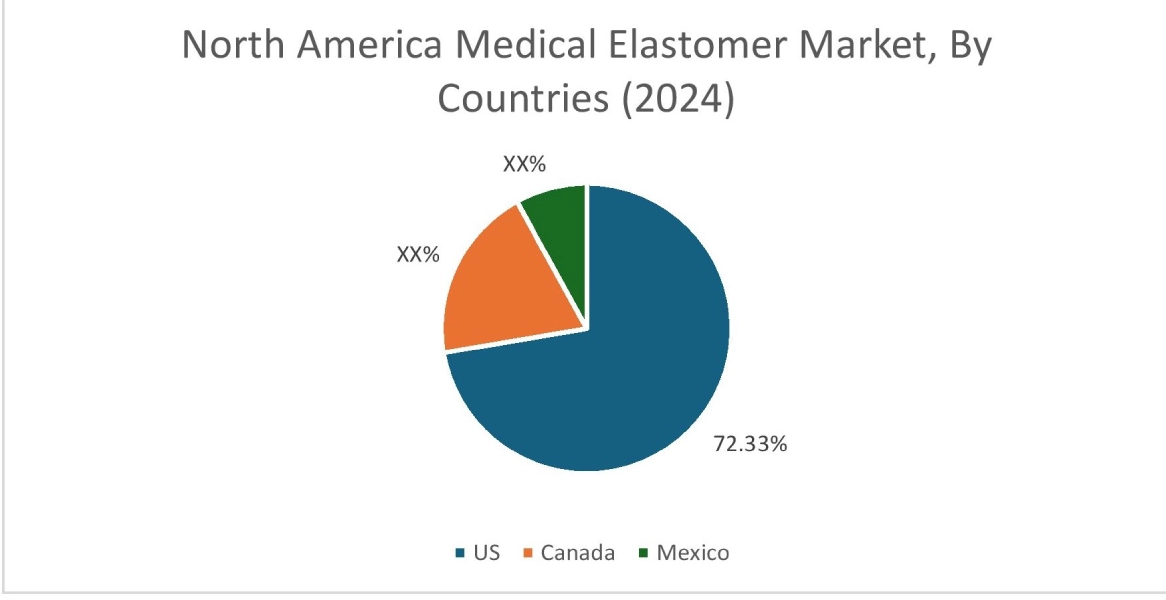
In 2024, North America accounted for the highest market share at 40.39% and was valued at USD 2.81 Billion and is expected to reach USD 5.05 Billion in 2032. In North America, the U.S. accounted for a market share of 72.33% during the base year of 2024. The upwards trajectory of regional share is primarily driven by highly advanced and well-established medical device sector. The presence of numerous leading medical device manufacturers, coupled with substantial investments in research and development, continuously drives the demand for innovative, high-performance elastomer solutions. This robust industry actively pushes for new material advancements to meet evolving needs in areas such as minimally invasive surgeries, sophisticated drug delivery systems, and a growing array of complex medical implants.
- For instance, according to MEDPAC, medical devices typically account for approximately 4% to 6% of the total healthcare expenditure in the United States
Thus, as per analysis, the region's strong focus on technological innovation and adherence to stringent regulatory standards collectively position North America as a key region for the market.
In Asia Pacific, the medical elastomers industry is experiencing the fastest growth with a CAGR of 12.5% over the forecast period owing to significant advancements in drug delivery systems and wearable devices. This region's growing healthcare infrastructure, rising healthcare expenditure, and increasingly health-conscious population are creating need for innovative medical technologies. Elastomers are crucial to these advancements, providing the necessary flexibility, biocompatibility, and durability for cutting-edge products such as wearable insulin injectors that offer precise, continuous, and user-friendly medication for chronic conditions such as diabetes. Furthermore, the rising popularity of various wearable medical devices relies heavily on medical-grade elastomers for comfortable, long-term skin contact and reliable performance. Collectively these factors fuel Asia Pacific medical elastomer market analysis.
European market is defined by region's stringent regulatory standards and a proactive focus on bio-based and sustainable elastomer solutions. Europe's strong commitment to sustainability is accelerating the adoption of bio-based and sustainable elastomers. This push for greener materials, derived from renewable resources and with a reduced carbon footprint, aligns with both regulatory pressures and growing consumer demand for environmentally responsible healthcare products, thereby creating substantial opportunities for innovation in this specialized sector. Hence, as per analysis, these factors collectively present a positive impact on the European medical elastomer market trends.
The market in Latin America is defined by increasing adoption of minimally invasive surgeries across the region. Countries like Brazil are at the forefront of this trend, driven by factors such as a growing geriatric population, rising prevalence of chronic diseases requiring surgical intervention, and an increasing patient preference for procedures offering benefits such as shorter hospital stays, reduced pain, and faster recovery times. The shift towards MIS necessitates high-performance, flexible, and biocompatible elastomers for critical devices such as advanced catheters, flexible tubing, and precision surgical instruments, further propelling medical elastomer market trends.
The market in Middle East and Africa is characterized by the shift towards disposable medical products. The region is increasingly emphasizing infection control and hygiene in healthcare settings, leading to a surge in the demand for single-use medical devices. This increased focus on preventing hospital-acquired infections and ensuring patient safety directly translates into a greater consumption of elastomers for these disposable applications. Governments in the region are making substantial investments in healthcare infrastructure and promoting modern medical practices, further fueling the need for single-use medical supplies.
Top Key Players and Market Share Insights:
The Global Medical Elastomer Market is highly competitive with major players providing products to the national and international markets. Key players are adopting several strategies in research and development (R&D) and product innovation to hold a strong position in the global Medical Elastomer Market. Key players in the Medical Elastomers industry include
- BASF SE (Germany)
- Trelleborg Group (Sweden)
- DuPont (U.S.)
- Dow (U.S.)
- Momentive Performance Materials Inc. (U.S.)
- Kuraray Co., Ltd. (Japan)
- Teknor Apex (U.S.)
- HEXPOL AB (Sweden)
- Kraton Corporation (U.S.)
- Biomerics (U.S.)
Recent Industry Developments :
Product launches
- In 2024, Teknor Apex expanded its Medalist portfolio of medical-grade thermoplastic elastomers (TPEs). These new grades are specifically engineered for biopharmaceutical tubing applications, addressing the demand from medical device manufacturers for high-quality, regulatory-compliant TPEs that offer flexibility and rubber-like elasticity.
- In 2022, HEXPOL TPE officially introduced its new line of medical thermoplastic elastomers featuring bio-attributed content, expanding its portfolio of materials derived from non-fossil feedstocks based on the mass balance principle.
Medical Elastomer Market Report Insights :
| Report Attributes | Report Details |
| Study Timeline | 2019-2032 |
| Market Size in 2032 | USD 14.22 Billion |
| CAGR (2025-2032) | 9.4% |
| By Type |
|
| By Application |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
How big is the Medical Elastomer Market? +
In 2024, the Medical Elastomer Market is USD 6.95 Billion.
Which is the fastest-growing region in the Medical Elastomer Market? +
Asia Pacific is the fastest-growing region in the Medical Elastomer Market.
What specific segmentation details are covered in the Medical Elastomer Market? +
By Type and Application segmentation details are covered in the Medical Elastomer Market.
Who are the major players in the Medical Elastomer Market? +
BASF SE (Germany), Trelleborg Group (Sweden), Kuraray Co., Ltd. (Japan), Teknor Apex (U.S.), HEXPOL AB (Sweden) are some of the major players in the market


